
Background:Gender-affirming hormone therapy is integral to the care of transgender individuals, but the hematologic complications of this therapy are not entirely understood. While secondary erythrocytosis is a widely recognized complication of testosterone administration, the exact prevalence of erythrocytosis in patients receiving exogenous testosterone for gender transition and the optimal management of this condition remain unclear. We performed a retrospective analysis of transgender individuals undergoing masculinizing therapy with testosterone at our institution to assess the prevalence of secondary erythrocytosis and review the management techniques utilized.
Methods:We performed a retrospective observational study of transgender individuals over the age of 18 years undergoing masculinizing therapy with exogenous testosterone between June 30, 2019 and June 30, 2020 at Oregon Health & Science University Hospital in Portland, Oregon. We collected data on average pre-testosterone hemoglobin and hematocrit values, formulation of testosterone and route of administration, peak hemoglobin and hematocrit values after the initiation of endocrine therapy, management of secondary erythrocytosis, and thrombotic events in those with secondary erythrocytosis. Descriptive statistics were employed to determine the prevalence of erythrocytosis using the reference ranges for cisgender males and to quantify average increase in hemoglobin and hematocrit levels from pre-therapy levels. Seaborn and Matplotlib libraries in Python were used for data visualization and pandas library in Python was used for statistical analysis.
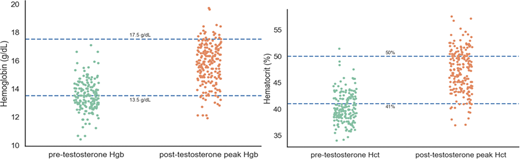
Results:A total of 234 individuals were included in this study with a mean age of 29 years. Testosterone cypionate was the most commonly administered formulation at 77.8% of cases, followed by transdermal testosterone formulations 14.5%, testosterone enanthate 4.2%, and combination transdermal testosterone plus cypionate 2.1%. Intramuscular injection was the most common route of administration at 65.4% followed by subcutaneous injection 15.8% and transdermal gel 12% and patch 2.1%. The mean pre-testosterone hemoglobin was 13.5 g/dL and hematocrit was 40.3%. After initiation of hormone therapy, the mean hemoglobin peak was 15.7 g/dL and hematocrit peak was 47.2% (Figures 1 and 2). It took an average of 21 months after initiation of therapy to reach peak hemoglobin and hematocrit levels. 23.5% of patients met the definition of erythrocytosis using our institutional cisgender male reference range for hematocrit above 50%, and 8.5% exceeded a hemoglobin threshold of 17.5 g/dL. Only one thrombotic event, a superficial venous thrombosis, occurred in those with secondary erythrocytosis. Dose reduction of testosterone was performed for 14.5% of patients who developed erythrocytosis, and no other management strategies, including therapeutic phlebotomy, were documented. 88.9% of patients with erythrocytosis had received testosterone cypionate, with other formulations infrequently associated with polycythemia-range hematocrit levels.
Discussion:Our analysis of transgender individuals undergoing masculinizing therapy with testosterone revealed an average increase in hemoglobin of 2.2 g/dL and in hematocrit of 6.9% after the onset of endocrine therapy. In total, 23.5% of patients met the threshold for secondary erythrocytosis using the hematocrit reference range for cisgender men after initiation of testosterone while only 8.5% met the hemoglobin threshold of 17.5 g/dL. The clinical consequences of this hematologic shift are unclear, with only one patient documented as developing a superficial venous thrombosis, and no occurrences of deep venous thrombosis, pulmonary embolism, or other thrombotic events. It also remains uncertain if utilizing the reference range for cisgender men is an appropriate method of predicting thrombotic risk in this population. The sole management strategy recorded was testosterone dose reduction as no patients had documentation of any therapeutic phlebotomy to lower their hematocrit levels. Of note, some providers recommended that their patients with erythrocytosis donate blood, which was not able to be documented in the medical record. Further studies are required to better define the clinical sequelae of erythrocytosis in this patient population and to determine optimal management of this condition.
Shatzel:Aronora, Inc.:Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal